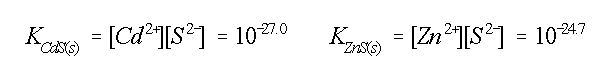9. Sorption kinetics of dissolved zinc and cadmium on harbor sediment suspended in oxic
seawater; a laboratory simulation.
Abstract
The results of sorption experiments of trace metals in
suspensions of harbour sediment with seawater are reported.
Anoxic sediment from the Rotterdam harbour was suspended in
oxic seawater to simulate the sorption kinetics of Cd and Zn
when harbour sediment is dumped at sea. During the simulated
suspension the dissolved concentrations of Zn, Cd and major
redox-sensitive element (Fe, Mn) were measured with fast
separation techniques. The redox potential (Eh) measurement
showed that oxidation of the anoxic sediment suspension can be
very fast when oxygen was added. This redox change was also
reflected in a rapid decrease of dissolved Fe, P and Mn in the
sediment slurry. The dissolved Cd concentration showed a rapid
decrease (0-10 minutes) during suspension. This was attributed
to: (1) sorption onto sulphides, (2) coprecipitation with Fe-
oxyhydroxides and/or (3) adsorption onto Fe-hydroxides. After
1 day Cd was released from the suspended matter which could
only be attributed to the oxidation of metal-sulphides. The
dissolved concentration of Zn in suspension showed similar
initial adsorption, but Zn showed no release after several
days. These experiments show the importance of scavenging when
anoxic harbour sediments, contaminated with heavy metals, are
dumped in oxic seawater.
9.1. Introduction
The port of Rotterdam (The Netherlands) is situated in
the upper estuary of the river Rhine and Meuse. Suspended
material carried by the rivers is deposited in this harbour
area. To maintain access to the harbours by bulk carriers and
supertankers the channels have to be dredged. About 23.106 m3
harbour sludge have to be removed each year. This sludge is
contaminated with organic and inorganic pollutants and the
major part is deposited at a designated area in the North Sea,
approximately 10 km off the coast of The Hague. The liberation
of pollutants may affect the quality of marine life at the
dumping site or in the coastal zone when fine particles are
carried with the water (VISSER et al., 1991). These problems
have been addressed in studies by GUSTAFSON, 1971, HALL, 1989,
STERN & GRANT, 1981, SNITZ et al., 1979, HIRST & ASTON, 1983,
TRAMONTANO & BOHLEN, 1984 and PRAUSE et al., 1985.
Speciation under anoxic environment. Sediments deposited
in the harbour have a high organic carbon content. Organic
carbon in the sediment will be degraded. A first step in the
degradation sequence is the consumption of oxygen (STUMM &
MORGAN, 1981). Oxygen is consumed rapidly in the toplayer of
the sediment, the underlying sediment will become oxygen
depleted. Changes between oxic and anoxic environment in
sediment cores occurs within the range of millimetres (DAVISON
et al., 1991, DAVISON et al., 1994). The succession of
reactions in the sediment is mainly reflected in the vertical
distribution of components (WILSON ET AL., 1986).
When anoxic harbour sludge is dumped into oxygenated
seawater the redox conditions will change. In general the
behaviour of heavy metals in aquatic systems is primarily
controlled by Eh (redox potential) and the pH (DAVIS & LECKIE,
1978, BENJAMIN & LECKIE, 1981, CHAROENCHAMRATCHEEP et al.,
1987, CALMANO, 1993). The sorption behaviour of heavy metals
depends on liquid speciation and on available sorption sites.
Usually trace metals are for a major part adsorbed on active
surface sites of inorganic and organic particles. Under anoxic
conditions sulphides can effectively bind Zn and Cd (LU &
CHEN, 1977). The reaction constants show that Cd is more
effectively bound by sulphide than Zn (STUMM & MORGAN, 1981):
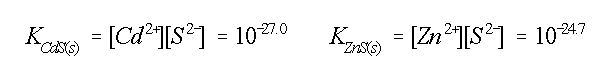
In pore water studies of anoxic sediment the dissolved Zn and
Cd concentration is almost zero which indicates that effective
adsorption sites are formed with a high affinity for heavy
metals (DAVIS-COLLEY et al., 1985, GIBLIN et al., 1986,
HUERTA-DIAZ & MORSE, 1992). WALLMANN (1990) calculated for
pore water systems that the concentration of non-sulphidic
complexes and minerals were not important for heavy metal
binding compared to sulphidic species. In marine and estuarine
sediments sulphides are not limited because seawater contains
sulphate which will diffuse into the sediment where it can be
reduced to sulphides. In anoxic sediments the redox-sensitive
elements like Fe and Mn will react to the thermodynamically
most favourable state, Fe(III)-species will change to Fe(II)
and Mn(IV) to Mn(II) species. In estuarine sediments the
organic carbon content is high enough to maintain anoxic
conditions.
Speciation under oxic conditions. In an oxic environment
the speciation is different compared to the speciation under
anoxic conditions. In oxic seawater at pH of 7.8 - 8.2 cadmium
chlorocomplexes are dominant species of dissolved Cd for zinc
the free ion and Zn-hydroxides are dominant (LU & CHEN, 1977,
COMANS & VAN DIJK, 1988, HEGEMAN et al., 1992). Trace metals
will adsorb to specific surface metaloxides. With constant Eh
and pH values almost linear adsorption isotherms between the
solid and liquid trace metal concentrations can be expected
(MOREL, 1983). Solid sulphide and dissolved sulphide species
are not stable, and will be oxidized in oxic seawater.
Suspension of anoxic sediments. When anoxic harbour
sediment is dumped into the oxic water the chemical equilibria
are disturbed. Divalent Fe in anoxic sediment will be oxidized
to trivalent Fe in oxic seawater and precipitated as Fe(OH)3
(STUMM & MORGAN, 1981, ROEKENS & VAN GRIEKEN, 1983). Fe(III)-
hydroxide has a high affinity for Cd and Zn. These freshly
formed amorph Fe-hydroxides have an enormous sorption capacity
(JENNNE, 1968) compared to the crystallized or aged
ironhydroxides (FULLER et al., 1993). In natural systems there
is a complete range of different Fe-precipitates (KUMA et al.,
1991) and the iron oxyhydroxides will age once they are
precipitated (BENJAMIN & LECKIE, 1981). The cations of Cd and
Zn can also coprecipitate with the Fe-hydroxides. Sorption and
coprecipitation will decrease the dissolved Cd and Zn
concentration. (SHIGEMATSU et al., 1975, FRANCIS & DODGE,
1990, NYFFELER et al., 1986). BOURG (1988) stated that in the
transition of anoxic to oxic conditions the following
consecutive reactions can be distinguished; (1) oxidation of
metal sulphides, followed by (2) the formation under oxic
conditions of new species such as dissolved chlorocomplexes in
marine and estuarine environments. Sequential extraction
studies of sediments showed that heavy metals associated with
the reduced phase (sulphides) in anoxic conditions were
transformed to the carbonaceous phase upon oxidation of the
sediment (STENEKER et al., 1987, RECKE & FÖRSTNER, 1986). The
Fe(III)-formation can be retarded by the formation of Fe(II)-
organic complexes where Fe(II) is an intermediate in the
oxidation of organic carbon in the oxic water (STUMM & MORGAN,
1981). Whereas a re-oxidation of Mn(II) is accomplished by
complex catalytic surface oxidation of Mn by ironoxides (SUNG
& MORGAN, 1981, VAN DER WEIJDEN, 1975).
Toxicity. In sediments the toxicity is dependent on the
availability of the metals. Mostly the free metal ion activity
is correlated with biological effects. DI TORO et al., (1992)
demonstrated the toxicity of cadmium when the ratio
[SEM]/[AVS] > 1. AVS (Acid Volatile Sulphide) represents a
sulphide fraction extracted with cold hydrochloric acid and
SEM represents the Simultaneously Extracted Metal
concentration. If the reactive pool of sulphide can no longer
keep the trace metals from solution the metals will become
available in solution. In general the key question is: will
the specie become available for biotic life? These suspension
experiments gave us some unexpected results in a non-
equilibrium system.
Sorption kinetics of heavy metals from anoxic to oxic
conditions are not understood completely. Therefore we
investigated the kinetic behaviour of dissolved Zn and Cd
during simulated dumping of anoxic harbour sediment in oxic
water. Simple laboratory experiments with fast separation
techniques showed the sorption processes during suspension of
anoxic harbour sediment in oxic seawater.
9.2. Material and methods
Sediment: Harbour sediment was collected from the Rotterdam
harbour area, located at the entrance of the Rhine river (Hook
of Holland). With a large boxcorer undisturbed sediment
samples could be obtained. The sediment was stored at 4 øC
until use.
Seawater: North Atlantic seawater (Salinity: 35 g.kg-1 or þ),
collected in as subsurface layer near the central Doggerbank
area, was diluted (1:6) with Milli-Q water (Millipore) to
obtain a salinity of 30 þ which is equal to the salinity at
the coastal dumping site.
Material: All handling material was treated with 0.1 M HCl for
several days and rinsed several times with Milli-Q water. In
sorption experiments with trace metals PFA (PolyFluorAlkoxy
polymer) vessels exhibited the least adsorption to the vessel
wall. The filter set (polyethylene and polyacetate) was tested
on adsorption of trace metals, no significant adsorption was
measured for Zn and Cd.
Redox potential measurements: A suspension of harbour sediment
was incubated in a glass three-neck round bottom flask for 4
weeks. The suspension was stirred with a magnetic stirrer in
the dark. The flask was sealed with air-tight septum caps. A
metal needle connected to a N2-flask which was pushed through
the septum kept an overpressure of nitrogen above the
suspension. Therefore oxygen could not penetrate the
suspension and experimental handling through the other
openings was possible. A Pt electrode with calomel reference
electrode was pushed gently into the stirred suspension. The
pH and the redox potential (Eh), calibrated with a Zobell
solution, were recorded continuously during 350 minutes. At
t=310 minutes the nitrogen purge was ended, the stopper was
removed, and air could diffuse into the slurry. Thirty minutes
later (t=340 minutes) we started to bubble air through the
suspension in order to maximize the contact between oxygen and
the suspension.
Suspension experiments: Undisturbed harbour sediment was used
to simulate the suspension of harbour sediment at sea. Small
subsamples of the sediment were taken with a half-open
polyethylene 11 cm3 syringe. The open end of the syringe, with
the piston downward, was placed gently on the sediment
surface. The piston was held and the syringe was pushed into
the sediment until the syringe was completely filled with the
wet sludge. The syringe was pulled out of the sediment and the
contents was immediately pushed into a well-stirred PFA vessel
filled with 700 cm3 seawater (S=30). From this suspension
subsamples were taken in order to determine the dissolved
elemental concentration. The sampling scheme is illustrated in
figure 1. It was essential to use a very quick sampling
technique to obtain a high resolution in time. Therefore
filtration with a high capacity filter (diameter: 47 mm, 0.45
æm, polyacetate, Schleicher & Schöll) was used. The filtrate
was acidified to pH=1 by addition of suprapur HNO3 (Merck) and
analyzed by ICP-AES (Induced Couple Plasma-Atomic Emission
Spectroscopy) for dissolved Fe, Mn, and P. High resolution
measurements of the trace elements Cd and Zn could not be made
by ICP-AES due to low concentrations and matrix effects. The
suspension concentration (m) was determined after the
subsamples were taken. To determine m a subsample of the
sediment suspension was washed three times with water,
centrifuged, dried at 105 øC and weighed.
Sorption of heavy metals during suspension: Sorption of Cd and
Zn during suspension was followed by the addition of the
metals spiked with radionuclides in diluted seawater (S=30 promille
at 20 øC. To avoid isotopic exchange reactions (ATKINSON et
al., 1971), solutions with a high concentration of Zn (1.0
mg/L) and Cd (1.0 mg/L) were prepared in a PFA vessels (total
volume: 700 cm3) and these were spiked with a high specific
activity 65Zn or 109Cd radionuclide. Before the suspension
experiment started, the gamma-activity of the solution was
determined in fivefold using 1 mL aliquots. The anoxic harbour
sediment was added in a similar fashion as described in the
previous section and an identical filtration scheme was
applied (fig. 1).
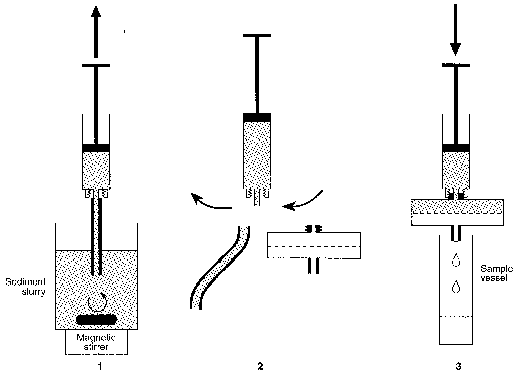
Figure 1.
Schematic representation of the quick sampling technique. The
experimental manipulations 1 - 3 were performed within 30
seconds.
(1) The suspended sediment is sucked through a small piece of
tubing into the syringe. (2) The tube is quickly removed and
replaced by a large filter holder (diameter: 47 mm) with Luer-
Lock connection. (3) The piston presses the suspension through
the filter until approximately 5 mL solution is collected in
the sample vessel.
In parallel experiments pre-oxidized sediments were used. The
same volume (11 cm3) of the anoxic sediment was suspended in a
50 mL seawater and clean air was bubbled through the
suspension for 24 hours. This pre-oxidized sediment was added
to the spiked seawater and the dissolved concentration of Zn
and Cd was measured in time. The pH was measured continuously.
Zn and Cd released from the sediment were less than 5 % of the
added concentration.
The gamma-activities (A) of the samples, corrected for
background activity, were determined by a multichannel counter
(LKW-Wallac) with NaI-crystal. The channel window included the
photopeak of the radionuclide. The fraction on the initial
concentration (F) in the dissolved phase at time t was
calculated according to the equation:
F(t) = A(t) * A(t=0)-1 .
9.3. Results and discussion
9.3.1. Redox potential during sediment oxidation
In fig. 2 the Eh is shown in a anoxic incubated sediment
suspension.
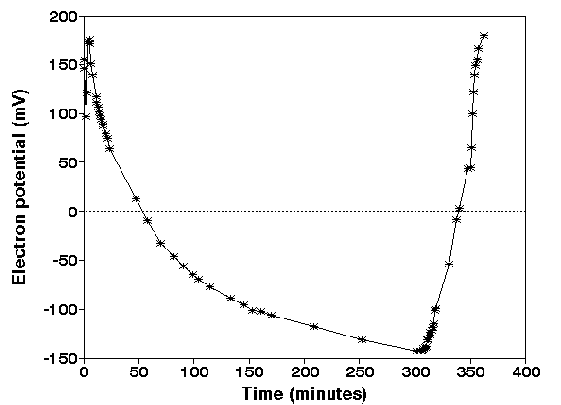
Figure 2.
Redox potential of incubated harbour sludge after
implementation of a Pt electrode. The sediment slurry in the
vessel was purged with nitrogen (t= 0-310 minutes), at t=310
nitrogen purging was inactivated and the stoppers were opened
and at t=340 minutes aeration with pressurized air was
started. Sediment/water= 1:10, pH = 8.5 ñ 0.1, Salinity = 30
þ.
The slow decrease of the redox potential from t=0
to 200 minutes is a result of a slow adaptation of the
electrodes to the anoxic environment. When oxygen was allowed
to contact the surface of the slurry at t= 310 minutes (fig.
2), the Eh increases almost immediately. The redox potential
increases much faster when air is bubbled through the slurry.
Although the measured Eh cannot be used to calculated the
actual thermodynamic state of the species present in the
suspension (MOREL, 1993), it is clear that reduced species can
be oxidized quickly when oxygen is present. This experiment
shows also that redox-related processes during the oxidation
of reduced sediments can be very fast. Therefore, rapid
separation techniques should be used to monitor dissolved
trace metals during the oxidation process. Similar Eh changes
can be expected when anoxic sediments are dumped in oxic
seawater. The fast initial sorption processes can only be
monitored when the time scale is within minutes.
In our experiment the colour of the suspension changes from
black (anoxic) to light-brown (oxic) within 24 hours. But the
colour of a suspension did not represent the Eh during
oxidation, the colour was still black when the redox potential
was at maximum.
9.3.2. Sorption kinetics of redox sensitive elements
In fig. 3 dissolved Fe, Mn and P concentrations are shown
after suspension of the harbour sludge in oxic water.
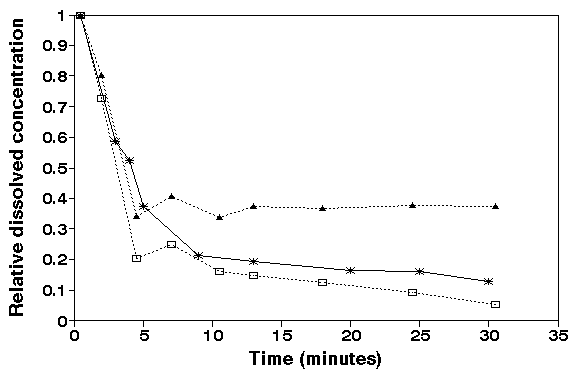
Figure 3.
Dissolved concentrations of iron, manganese and phosphorous
versus suspension time. The concentration is expressed as a
fraction of the concentration at t = 0.5 minutes. At t=0
harbour sediment was added and kept in suspension. Initial
concentration (t=0.5 min.): Fe 0.74 mg/L, P 2.1 mg/L and Mn
0.56 mg/L. Suspension concentration: 5 g/L, pH= 7.5 - 7.7,
Salinity = 30 promille.
The
redox sensitive elements (Fe, Mn) disappear within minutes,
similar to the change of the redox potential in the suspension
(fig. 2). It is obvious that we would have missed the rapid
decrease in concentrations when we had not used a fast
separation technique. Instead we would have observed a
constant concentrations.
The quick decrease of dissolved Fe represents the
precipitation of Fe-hydroxide. Dissolved phosphorous (ortho-
phosphate) has the same pattern as dissolved iron because
phosphate is strongly sorbed on reactive ironhydroxides.
Freshly precipitated ironhydroxides have a high surface area
and a high sorption capacity (ATKINSON et al., 1967, JENNE,
1968, BALISTRIERI & MURRAY, 1979). Once formed in the oxic
water iron hydroxide is relative stable and the dissolution
rate is low at pH values between 7.0 and 8.2 (KUMA et al.,
1993, PATRICK & HENDERSON, 1981). The decrease of dissolved Mn
can be attributed to the oxidation of Mn2+ and precipitation as
MnOx (2ó x ó4).
During the suspension sulphides, present in the anoxic
estuarine sediment, can be oxidized and protons are produced.
In our studies a decrease of the pH was not observed. The
suspension has a high Acid Neutralization Capacity (ANC)
because the Rotterdam harbour sediment has a high CaCO3 content
and the pH will therefore remain constant. CALMANO et al.,
(1993) showed the importance of the Acid Neutralization
Capacity (ANC) of suspended anoxic sediment under oxic
conditions. A decrease of the pH from 8 to pH=3-4 will have
major consequence for the behaviour of trace metals (DAVIS &
LECKIE, 1978).
The observed decrease of the dissolved concentrations
(fig 3.) with suspended harbour sediment has not been shown
before with a resolution time of 0.5 minutes.
The redox-sensitive elements decrease in the same time
interval as the increase of the redox potential. Similar fast
oxidation processes will occur when anoxic estuarine sediment
is dumped in the oxic water or the anoxic bottom sediment is
resuspended during storm conditions. Induced by redox
processes new sorption sites of iron and manganese oxides are
formed.
9.3.3. Trace element kinetics during suspension
Cadmium. Figure 4 shows the decline of the dissolved Cd
concentration when anoxic and pre-oxidized sediment was
suspended in oxic seawater.
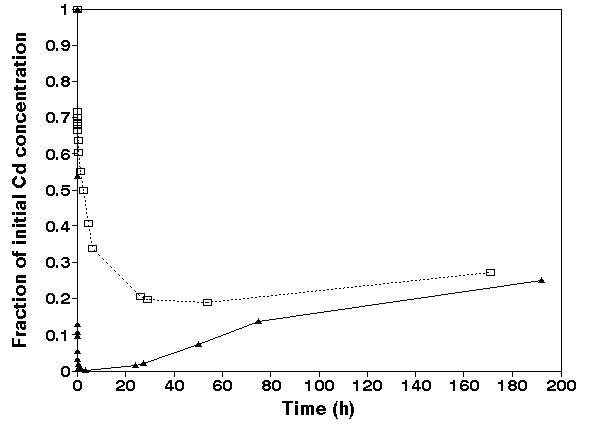
Figure 4.
Adsorption of cadmium expressed as the initial concentration
of Cd in solution versus contact time in sediment-seawater
suspension. Anoxic sediment ( �, initial concentration: 1
mg/L, m = 3.2 g/L ) and oxic sediment (open square, initial
Cd concentration: 1 mg/L, m= 4.2 g/L, pH= 7.9 ñ 0.1), Salinity
= 30 promille.
The dissolved Cd concentration
shows a rapid decrease when anoxic sediment was suspended.
After one day partial release of Cd from the sediment to the
dissolved phase is observed. The pre-oxidized sediment showed
a different sorption behaviour. Although the sediment
composition was identical the sorption kinetics depend on the
oxidation state of the sediment. The sorption kinetics of the
oxidized sediment was similar to the behaviour observed for Cd
on suspended matter from the River Rhine (COMANS & VAN DIJK,
1988). A rapid initial adsorption within a few hours is
followed by a slower adsorption during several days. The
sorption on the anoxic sediment showed a very strong
adsorption followed by a partial desorption after a few days.
When the sorption experiment is followed for several days the
anoxic sediment showed pseudo-equilibrium; a similar
distribution as the pre-oxidized sediment. This is shown in
fig. 4 when the suspension time was higher than 4 days. The
observed kinetics in the anoxic sediment can be explained by;
(1) adsorption to non-oxidized sulphides (2) coprecipitation
with freshly formed iron(hydr)oxides and (3) adsorption on the
iron(hydr)oxides. The solid sulphides species have a very high
affinity for heavy metals (DAVIES-COLLEY et al., 1985, HUERTA-
DIAZ & MORSE, 1992).
The oxidation of sulphides to sulphate occurs slower than the
sorption of Cd on the sulphide-sites. With increasing contact
time of the sulphide minerals and oxygenated water these
minerals will be oxidized and the adsorbed trace metals
released. The liberated Cd will then be adsorbed on other
existing or newly formed sorption sites on the suspended
matter. Freshly formed Fe and Mn oxyhydroxides have a high
specific surface area and can adsorb a large amount of
dissolved trace metals. With increasing amounts of host phases
the dissolved trace-metal concentrations will decrease. The
freshly formed Fe and Mn oxyhydroxides are not stable and will
start to age. Therefore, adsorbed trace metals may become
partly released.
Dissolved heavy metals can also be scavenged from solution by
coprecipitation with Fe or Mn oxyhydroxides. In that case the
heavy metals will be more tightly held by the substrate and
will, upon aging, more readily become incorporated in the
lattice of the host phase.
The experiments (Fig. 4) show that the initial sorption
capacity of the anoxic suspended matter is higher than the
capacity of the pre-oxidized suspended matter. After about 24
hours the capacities of the two suspensions start to become
equal which is completed after about a week. We ascribed that
to the above mentioned sequence of sorption on sulphide
phases. Subsequent release and sorption on freshly formed Fe
and Mn oxyhydroxides and further release upon aging of these
host phases.
In the experiments with Cd, sulphidic species have the major
adsorption capacity and scavenge the heavy metals from
solution. When oxidized Cd will be release, the amount
released is approximately equal the amount which was scavenged
by sulphides. This can be conclude from the pre-oxidized
sediment which has the same concentration when time was higher
than 5 days.
Zinc. Figure 5 shows the fast decrease in dissolved
concentrations of Zn after addition of the suspended harbour
sludge.
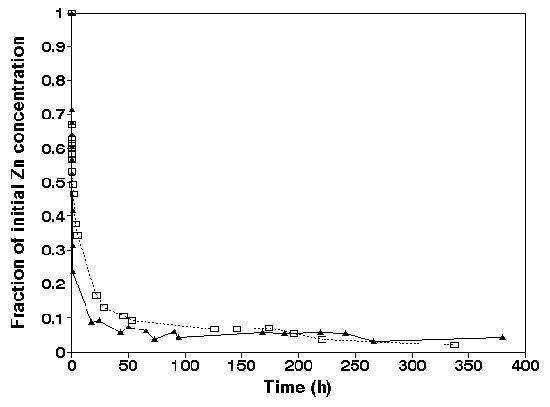
Figure 5.
Adsorption of zinc expressed as the initial concentration of
Zn in solution versus the contact time in sediment-seawater
suspension. Anoxic sediment ( �, initial concentration: 1
mg/L, m = 1.6 g/L ) and oxic sediment (open square, initial
concentration: 1 mg/L, m= 0.92 g/L, pH= 8.0 ñ 0.2), Salinity =
30 promille.
A pseudo-equilibrium is reached in 15 minutes similar
as for Cd. In the experiment where anoxic harbour sludge was
used the sorption was higher than in the pre-oxidized
suspension. Although the suspension concentration of the pre-
oxidized suspension was denser (1.6 g/L) the sorption was less
than in the experiments were anoxic added sediments (0.92
g/L). Therefore, the anoxic added suspension must have a
higher sorption capacity. This indicates that the anoxic
sediment had very reactive sorption sites with a high affinity
for zinc. We attribute this affinity due to sulphides which
were not immediately oxidized when anoxic sediment was dumped.
In contrast to Cd, Zn was not released after 24 hour. This can
be explained by a high affinity of Zn for ironhydroxides which
are formed during oxidation of the sediment. A second reason
is that Zn-sulphides have a very high oxidizing rate (LU &
CHEN, 1977) and therefore will not be released easily. Zn has
a higher affinity for sorption sites on solid ironoxides than
Cd in seawater suspensions (VAN DER WEIJDEN, 1975).
Suspended anoxic sediments have a high scavenging
capacity of heavy metals. These results show that separation
must be fast in order to show the kinetic effect of heavy
metals during simulated in oxic sediment. Pseudo-equilibrium
for the sorption of Cd and Zn on suspended sediment is
attained within 15 minutes.
Anoxic harbour sediments which are dumped in seawater can
immediately scavenge heavy metals from the dissolved phase.
When harbour sediment is immediately redeposited on the bottom
no harm will be done to the aquatic environment because anoxic
conditions will be re-established and the sediment will be
covered by an oxic blanket containing sorption sites in the
form of ironhydroxides (THOMSON et al., 1993). If the
suspended sediment is kept in suspension heavy metals can be
partially released when the sulphide phase is oxidized. In the
marine environment, the sediment suspension will be diluted
and establish a new equilibrium with the seawater which is low
in trace-metals. The heavy metals on the suspended sediment
will be released continuously to the water phase where it
becomes available to biotic life.
9.4. Conclusions
With simple laboratory experiments the sorption of cadmium and
zinc was simulated when anoxic sediment is suspended in oxic
seawater. Parallel studies of the redox potential, the
sorption behaviour of Fe, Mn, Cd and Zn during suspension gave
evidence for the chemical processes during dumping. The redox
potential increased rapidly when oxygen could enter the anoxic
suspension. The dissolved concentration of the redox sensitive
elements Fe and Mn and of P decreased rapidly when anoxic
sediment was suspended. Dissolved Cd and Zn were scavenged
from solution when anoxic sediment was suspended. The
scavenging of Cd was attributed to a combined interaction of:
(1) sorption to non-oxidized sulphides, (2) (co)precipitation
of Fe2+, (3) adsorption to freshly precipitated
Fe(oxy)hydroxides. The sorption to mineral sulphides is
considered as the main contribution of the rapid decrease in
cadmium. For Zn strong adsorption was observed on anoxic
sediment and this was attributed to adsorption to sulphides
and precipitated ironhydroxides.
If the anoxic sediments can settle in coastal water, Zn and Cd
are captured by the sediment. These trace metals can be
released to the water phase when the sediment is kept in
suspension in the oxic seawater.
Registry No. Cd, 7440-43-9; Cd2+ ,22537-48-0, CdS, 1306-23-6;
Zn, 7440-66-6, Zn2+, 23713-49-7, ZnS, 1314-98-3; Fe, 7439-89-
6, Fe2+, 15438-31-0, Fe3+, 20074-52-6, Mn, 7439-96-5, Mn2+,
16397-91-4, Mn4+, 19768-33-3, S2-, 18496-25-8.
9.5. REFERENCES
ATKINSON R., POSNER, A. & J. QUIRK, 1967. Adsorption of
potential determining ions at the ferric oxide aqueous
electrolyte surface. J. Phys. Chem. 71, 550-558.
ATKINSON, R.J., POSNER, A.M. & J.P. QUIRK, 1971. Kinetic of
heterogeneous isotopic exchange reactions: derivation of an
Elovich equation. Proc. R. Soc. Lond. A. 323, 247-256.
BALISTRIERI L. & J.W. MURRY, 1979. Surface of Goethite
(àFeOOH) in seawater. In: E.A. Jenne: Chemical modelling in
aqueous systems. ACS pp 275-298.
BENJAMIN, M.M. & J.O. LECKIE, 1981. Multiple-site adsorption
of Cd, Cu, Zn and Pb on amorphous iron oxyhydroxide. J.
Colloid Interface Sci. 79: 209-221.
BOURG A.C.M. 1988. Metals in aquatic and terrestrial systems:
sorption, speciation, and mobilization. p3-32 In: Salomons W.
and Förstner U.(eds) Chemistry and biology of solid waste.
Dredged material and mine tailings. Springer-Verlag 1988 pp
305.
COMANS, R.N.J. & C.P.J. VAN DIJK, 1988. Role of complexation
processes in cadmium mobilization during estuarine mixing.
Nature, 336: 151-154.
CALMANO, W., HONG, J., U. FöRSTNER, 1993. Binding and
mobilization of heavy metals in contaminated sediments
affected by pH and redox potential. In: IAWQ, First
International Specialized Conference on contaminated Aquatic
Systems: Historical Records, Environmental Impact and
Remediation. Milwaukee, Wisconsin, USA, June 14-16, 1993 pp
306-316.
CHAROENCHAMRATCHEEP, C., SMITH, C.J., SATAWATHANANONT, S.,
PATRICK, W.H., JR 1987. Reduction and oxidation of acid
sulphate soils of Thailand. Soil Sci. Soc. Am. J. 51, 630-634.
DAVIES-COLLEY, R.J., NELSON, P.O., WILLIAMSON, J., 1985.
Sulphide control of cadmium and copper concentrations in
anaerobic estuarine sediments. Mar. Chem. 16, 173-186.
DAVIS, J.A., LECKIE, J.E., 1978. Surface ionization and
complexation at the oxide/water interface. II. Surface
properties of amorphous iron oxyhydroxide and adsorption of
metal ions. J. Colloid Interface Sci. 67, 90-107.
DAVISON, W., GRIME, G.W., MORGAN, J.A.W., CLARKE, K. 1991.
Distribution of dissolved iron in sediment pore water at
submillimetre resolution. Nature 352, 323-324.
DAVISON, W., ZHANG, H., GRIME, G.W. Performance
characteristics of gel probes used for measuring the chemistry
of pore waters. Environ. Sci. Technol. 28, 1623-1632.
DI TORO, D.M., MAHONY, J.D., HANSEN D.J., SCOTT, K.J.,
CARLSON, A.R. & ANKLEY, G.T., 1992. Acid volatile sulphide
predicts the acute toxicity of cadmium and nickel in
sediments. Environ. Sci. Technol. 26, 96-101.
FEELY, R.A., FREFRY, J.H., MASSOTH, G.J., METZ, S., 1991. A
comparison of the scavenging of phosphorus and arsenic from
seawater by hydrothermal iron oxyhydroxides in the Atlantic
and Pacific Oceans. Deep-Sea Res. 38, 617-623.
FRANCIS, A.J. DODGE .C.J. 1990. Anaerobic microbial
remobilization of toxic metals coprecipitated with ironoxide.
Environm. Sci. Technol. 24, 373-378
FULLER, C.C., DAVIS, J.A., WAYCHUNAS, G.A., 1993. Surface
chemistry of ferrihydrate: Part 2. Kinetics of arsenate
adsorption and coprecipitation. Geochim. Cosmochim. Acta. 57,
2271-2282.
GIBLIN, A.E., LUTHER, G.W. & VALIELA, I., 1986. Trace metal
solubility in salt marsh sediments contaminated with sewage
sludge. Estuar. Coast. Shelf Sci., 23, 477-498.
GUSTAFSON, J.F., 1972. Beneficial effects of dredging
turbidity. World Dredging Mar. Const. 9, 44-72.
HALL, L.A. 1989. The effects of dredging and reclamation on
metal levels in water and sediments from an estuarine
environment off Trinidad, West Indies. Environ. Poll. 56,
189-207.
HEGEMAN, W.J.M., C.H. VAN DER WEIJDEN, ZWOLSMAN, J.J.G., 1992
Sorption of zinc on suspended particles along a salinity
gradient: a laboratory study using illite and suspended matter
from the river Rhine. Neth. J. Sea Res. 26, 285-292.
HIRST J.M. & S.R. ASTON, 1983. Behaviour of copper, zinc, iron
and manganese during experimental resuspension and reoxidation
of polluted anoxic sediments. Estuarine, Coastal and Shelf
Science 16, 549-558.
HUERTA-DIAZ, M.A. & J.W. MORSE, 1992. Pyritization of trace
metals in anoxic marine sediments. Geochim. Cosmochim. Act.
56, 2681-2702.
JENNE, E.A., 1968. Controls on Mn, Fe, Co, Ni, Cu and Zn
concentration in soils and water: The significant role of
hydrous Mn and Fe oxides. Am. Chem. Soc. Symp. Ser. 93: 337-
387
KUMA, K., SUZUKI, Y., MATSUNAGA, K., (1993). Solubility and
dissolution rate of colloidal gamma-FeOOH in seawater. Wat.
Res. 27, 651-657.
LU, C.S.J. & K.Y. CHEN, 1977. Migration of trace metals in
interfaces of seawater and polluted surficial sediments.
Environ. Sci. Technol. 11 1977 174-182
MOREL, F.M.M. 1983. Principles of aquatic Chemistry. John
Wiley & Sons, New York, pp 446.
NYFFELER, .U.P., SANTSCHI .P.H.LI .Y.H. 1986. The relevance of
scavenging kinetics to modeling of sediment-water interactions
in natural waters. Limnol. Oceanogr. 31, 277-292.
PATRICK W.H. JR, WILLIAMS B.G. & J.T. MORAGHAN, 1973. A simple
system for controlling redox potential and pH in soil
suspensions. Soil Sci. Soc. Amer. Proc. 37, 331-332.
PATRICK W.H. JR, & KHALID R.A., 1974. Phosphate release and
sorption by soils and sediments: effect of aerobic and
anaerobic conditions. Science, 186, 53-55.
PATRICK, W.H. & R.E. HENDERSON, 1981. Reduction and
reoxidation cycles of manganese and iron in flooded soil and
in water solution. Soil. Sci. Soc. Am. J., 45, 855-859.
PRAUSE B., REHM E., SCHULZ-BALDES M., 1985. The remobilization
of Pb and Cd from contaminated dredge spoil after dumping in
the marine environment. Environmental Technology Letters. 6,
261-266.
RECKE, M., & U. FÖRSTNER, 1986. Oxidation increases mobility
of toxic metals in dredged sludges. Chem. Environ., Proc. Int.
Conf.London, Selper, 809-818.
ROEKENS, .E.J., VAN GRIEKEN .R.E. 1983. Kinetics of
iron(II)oxidation in seawater of various pH. Mar. Chem. 13,
195-202.
SHIGEMATSU T., OMORI T., AOKI T. & M. MATSUI 1975.
Coprecipitation behavior of zinc in the oxygenation process of
ferrous iron. Bull. Int. Chem. Res., Kyoto Univ. 53, 435-433.
SNITZ, F.L., WEBER, W.J. JR., BARNEY, J.L., POSNER, J.C.,
1979. Effect of calcium and sediment concentrations on the
release of metals and nutrients from dredge spoil dispersions.
Aquat. Toxicol. Boek, 322-341.
STENEKER R.C.H., VAN DER SLOOT H.A., DAS H.A. 1987, Leaching
studies on dredged material in oxidized and reduced stat.
Report: ECN-87-092 Netherlands Energy Research Foundation,
P.O. BOX 1, 1755 ZG Petten, NL.
STERN, D.A. & GRANT .C.L. 1981. A laboratory investigation of
heavy metal adsorption on man dredge spills. Bull.Environ.
Contamin. Toxicol. 26, 213-218.
TRAMONTANO, J.M. & W.F. BOHLEN, 1984. The nutrient and trace
metal geochemistry of a dredge plume. Estuarine, Coastal an
Shelf Science. 18, 385-401.
STUMM. W. & J.J. MORGAN, 1981. Oxidation and Reduction In:
STUMM, W. & J.J. MORGAN. Aquatic Chemistry. John Wiley & Sons,
Inc., New York: p 418-503 Chpt. 7.
SUNG, W. & MORGAN .J.J., 1981. Oxidation removal of MnII from
solution catalysed by the gamma-FeOOH(dipidocrocite)surface.
Geochim. Cosmochim. Acta 45, 2377-2383.
THOMSON, J., HIGGS, N.C., CROUDACE, W., COLLEY, S. & D.J.
HYDES, 1993. Redox zonation of elements at an oxic/post-oxic
boundary in deep-sea sediments. Geochim. Cosmochim. Acta 57,
579-595.
TURNER, D.R., WHITFIELD, M. & A.G. DICKSON, 1981. The
equilibrium speciation of dissolved components in freshwater
and seawater at 25oC and 1 atm pressure. Geochim. Cosmochim.
Acta, 45: 855-881.
VAN DER WEIJDEN, C.H. 1975. Sorption experiments relevant to
the geochemistry of Manganese nodules. Ph.D Thesis, University
of Utrecht. 154 pp.
VISSER, M., DE RUIJTER, W.P.M., POSTMA, L. (1991). The
distribution of suspended matter in the Dutch coastal zone.
Neth. J. Sea Res. 27, 127-143.
WALLMANN K. 1990. Die Frühdiagenes und ihr Einfluss auf die
Mobilität der Spurenelemente As, Cd, Co, Cu, Ni, Pb und Zn in
Sediment- und Schwebstoff-Suspensionen. Ph.D Thesis, Hamburg.
WILSON, T.S.R., THOMSON, J., HYDES, D.J., COLLEY, S., CULKIN,
F., SORENSEN, J., 1986. Oxidation fronts in pelagic sediments:
diagenetic formation of metal-rich layers. Science 232, 972-
975.



Titel rapport: